
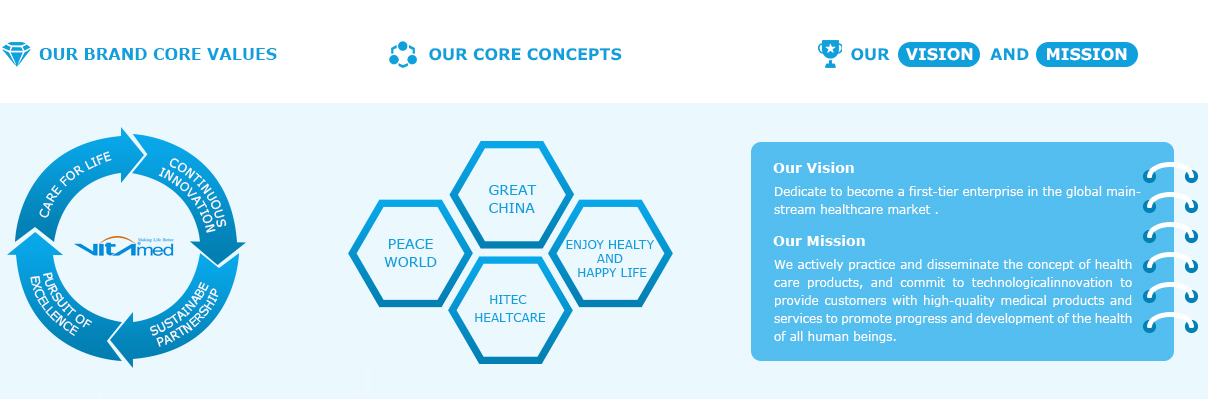
ABOUT US
Established in 2007 in Shanghai, China, Vitaimed is a leading medical device developer and manufacturer dedicated to advancing healthcare worldwide by developing, manufacturing, and marketing medical products that help improve the safety and effectiveness of patient care. Vitaimed product and service portfolio includes urological continence care, ostomy care, wound care, gynecological care, enteral feeding and irrigation products, and a full-service contract manufacturing business. Vitaimed products are with ISO13485, CE and FDA certificate. Vitaimed products are distributed in the United States, Germany, Greece, Italy, France, Spain, Portugal, Poland, CZ, Singapore, Thailand, Malaysia, Egypt, U.A.E., Korea, Brazil, Chile, Panama, S.Africa, Mozambique, and more than 40 other countries worldwide.
COMPANY CULTURE
Groth Path
2003
2007
2008
2011
2012
2013
2014
2016
2017
2019
R&D CENTER
R&D Center
R&D System
Vitaimed has formed and internationalized R&D structure, enhanced its strong R&D capabilities, and established an interactive and integrated R&D system in China, the Germany, etc.
At the end of 2019 ,Vitaimed had a total of 25 projects: 8 ostomy care, 5 biological innovative materials, 5 continence care can meet international standards,3 Urology care coating technology, and 2 wound and skin care and 2 electronic hospital and caring bed.

QUALITY POLICY
Vitaimed is committed to customer satisfaction and patient well-being.
All products and services provided to our customers shall be safe and effective for their intended use.
Each product and service shall be designed, manufactured, marketed, and delivered in compliance with all applicable laws, regulations, and standards.
Product quality will be accomplished through the consistent implementation and maintenance of an effective Quality Management System.
Every employee is responsible for the active promotion and the realization of Vitaimed quality policy within their job functions.
VITAIMED's quality philosophy is to achieve product perfection and customer satisfaction through the implementation and maintenance of an effective, stringent, and rigorous quality assurance system. Quality assurance plays a key role in the production of medical devices in partnership with full adherence to Good Manufacturing Practices (GMP) on a daily basis.
VITAIMED products are manufactured in strict accordance with the GMP Regulations and ISO standards. All parts, components, and materials used in manufacturing are received, stored, and handled in a manner consistent with our high quality standards. VITAIMED products are manufactured, assembled and packaged in air-filtered, temperature-controlled clean rooms, free from contamination. To ensure uniform environmental conditions at our manufacturing sites, temperature, humidity, air pressure, air filtration, and lighting are all strictly controlled. The environmental control system is inspected on a regular basis. Personnel sanitation rules are strictly enforced, and sanitized clothing is required for entrance to clean rooms. Measures are taken to prevent contamination of equipment, components, and finished products by cleaning agents and other chemicals. VITAIMED products are stored in clean, well-maintained warehouses.



VITAIMED's quality assurance program is founded on the belief that quality has to be built in from product conception, and ensured through raw material testing, in-progress production controls and end-product inspection. In brief , this program consists of the following procedures:
1. Review of product production records;
2. Approval of parts, components, packaging materials, labeling, and finished product;
3. Approval of product manufactured, processed, packaged;
4. Audits of the quality assurance program to verify compliance with the quality assurance program.
All quality audits are performed by trained personnel not having direct responsibilities for the products being audited. Audits results are documented in written audit reports, and reviewed by management responsible for the products audited. Follow-up corrective action, including re-audit of observed deficiencies, is taken when indicated.
VITAIMED values its commitment to quality and prides itself on its quality assurance system.
QA & RA Strategy
Maintain FDA QSR/cGMP compliance.
Maintain ISO 13485 & MDD certification.
Obtain CMD/CAS ISO 13485 certification.
Establish higher quality standards in world-class manufacturing.
Prevent & reduce product deficiencies from R&D through distribution.
Move toward six-sigma quality.
PRODUCTION CAPACITIES
VITAIMED is avertically integrated company with the following features:
ISO13485 certification
CE certification
FDA registration
Strict GMP compliance
State-of-the-art manufacturing equipment
Over 0.5million square feet of manufacturing space
Medical class cleanrooms(Class 10,000 and Class 100,000)
A well trained and highly skilled work force, over 1000 employees
A talented engineering and R&D team
An experienced management team with strong commitment to quality, excellence, and service.

VITAIMED's manufacturing capabilities include, but not limited to:
Tubing molding
Injection molding
Insert molding
Blow molding
Radio frequency(RF) sealing
Ultra sonic welding
Aseptic filling
Assembly in cleanrooms
Form fill seal packaging
Laboratory testing
Device design & development
Reverse engineering
ETO sterilization

Name: Catherine
Phone: +86 215 8399 546
Mobile: +86 139 1863 8349
Email: [email protected]
WhatsApp: +86 139 1863 8349